Menu
Iron Metal is one of the most used metals in industries and daily lives. We at MetalsTek Engineering, are renowned for our unwavering commitment to delivering top-quality high-purity Iron Metals, such as Iron evaporation material, sputtering target, wire, rod, tube, cubes, pellets, powders, setting the industry standard for reliability and excellence. We also have rich experience in custom Iron Metal products.

Material: Iron
Purities: 99.8%, 99.9%, 99.95%, 99.98%, 99.99%, 99.995%(80ppm)
Shape: Pieces, Granules, Ingots, or Customized
Size: Tailored Sizes
*Can be Used as Evaporation Material
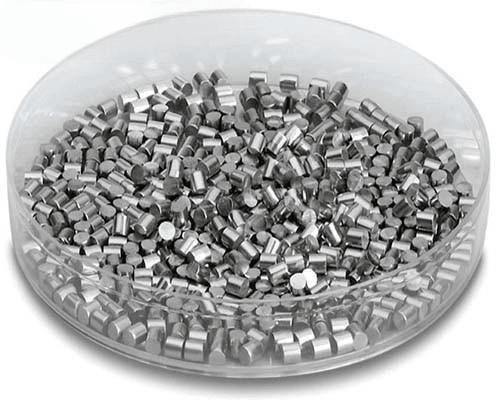
Material: Iron, 99.9%~99.99%
Form: Pellets, Pieces, Powder, Customized Shapes
Size: 1~6mm, or Tailored Size
Applications: Deposition Process, CVD, PVD, Optics Coatings, Displays

Material: Iron, 99.9%~99.95%
Shape: Discs, Plates, Column Targets, Step Targets, Custom-made
Sizes: Disc – Dia. 1”~14” * Thick 0.125” / 0.25”
Block – Thick≥1mm * Width ≤14” * Length≤32”

Material: Iron, 99%~99.995%, or Iron Alloy, Carbon Steel
Shape: Wire, Cable, Shaft, or Custom-made
Sizes: Diameter 0.1~5mm Wire, Can be Customized






Material: Iron, 99%~99.995%, or Iron Alloy, Carbon Steel
Shape: Disc, Cylinder, Rod, Shaft, Bar, or Custom Shapes
Sizes: Diameter 3~100mm, Can be Customized, Cut at Will

Material: Iron, 99%~99.995%, or Iron Alloy, Carbon Steel
Shape: Cube, Bar, Block, Square Bar, Hex Bar, or Custom Shapes
Sizes: Thickness>3mm, Can be Customized, Cut at Will
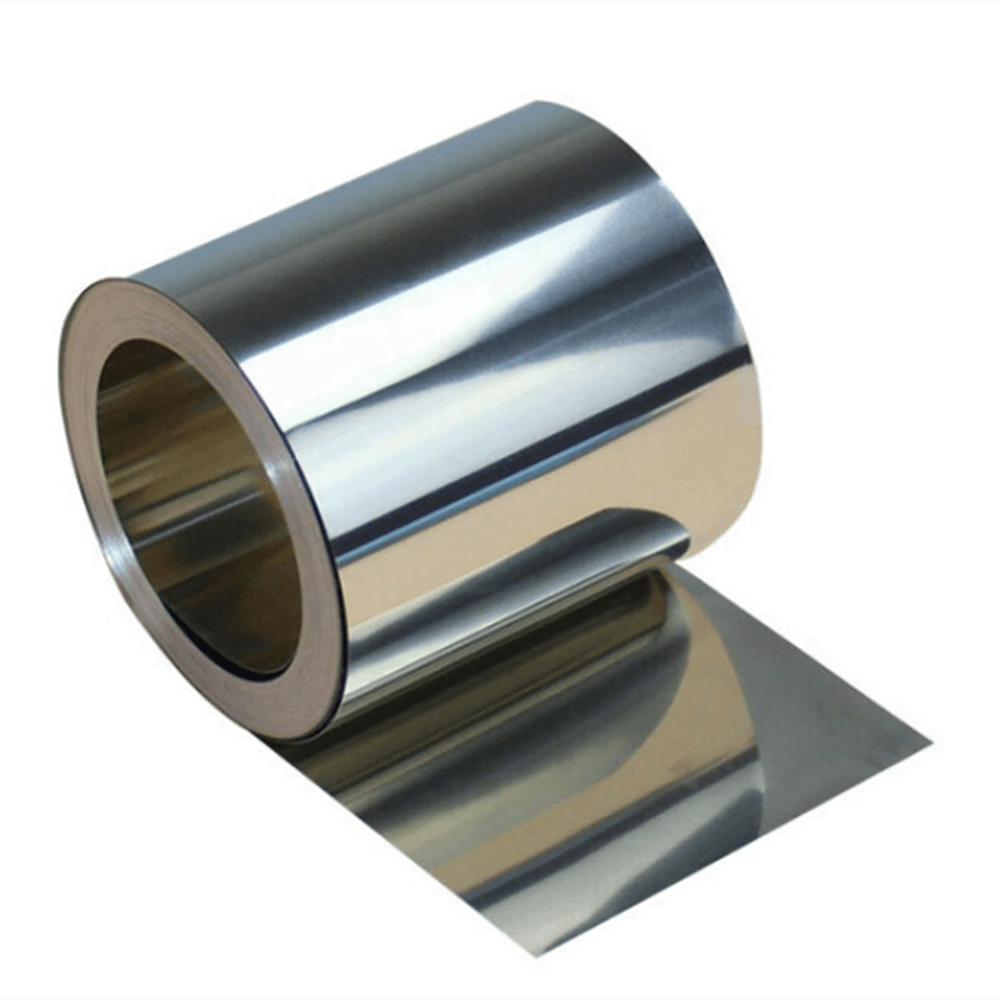
Material: Iron, 99%~99.995%, or Iron Alloy, Carbon Steel
Shape: Foil, Ribbon, Strip, Band, Sheet, Plate, Bar, or Custom Shapes
Sizes: Thickness>0.02mm, Width and Length Can be Customized, Cut at Will

Material: Iron
Purities: 99.99%
Shape: Cubic
Size: 10mm, 20mm, 25.4mm, 30mm, 50mm, or Tailored Sizes
Applications: Element Collections, Displays, DIYs, Metallurgy, etc.

Material: Iron
Purities: 99 %~99.995%
Shape: Spherical Powder, Nano Powder, Micro Powder, Carbonyl Powder
Size: Various Sizes, Tailored Sizes

Material: Iron, Fe
Purities: 99%~99.995%
Shape: Wire, Rod, Foil, Sheet, Plate, Bar, etc
Size: Tailored Sizes

Material: Iron Oxide, 99.9%
Properties: ≈1,538°C M.P., 5.18g/cc Density
Form: Pellets, Pieces, Powder, Customized Shapes
Size: 1~6mm, or Tailored Size
Iron is a chemical element with the symbol Fe and atomic number 26. It is a metallic-gray metal that belongs to the first transition series and group 8 of the periodic table. Iron is the most used metal globally, forming much of Earth’s outer and inner core and being the fourth most common element in the Earth’s crust. It has a melting point of 1,535°C, a density of 7.86 g/cc, and exhibits ferromagnetic properties. Iron is extensively used in various products like tools, automobiles, and machinery. When alloyed with carbon, it forms steel, a crucial material in building construction and automobile manufacturing. Additionally, Iron metal plays a vital biological role in carrying oxygen in blood and is evaporated under vacuum for applications in semiconductor production, magnetic storage media, and fuel cells.
The applications of Iron Metal are extensive and diverse, spanning various industries and sectors. Here are some typical applications based on the provided sources:
These applications underscore the versatility and importance of iron metal across industries ranging from manufacturing and construction to biomedicine and catalysis
Our Iron Metals are clearly labeled externally to ensure efficient identification and quality control. We take great care to prevent any damage during storage or transportation.
Iron is a metal that is found abundantly on Earth. It is used in various industries and is an essential element for the functioning of our modern society. However, many people do not fully understand what iron is, why it is so important, what iron is good for, and the difference between iron and steel. In this article, you will find the answers.
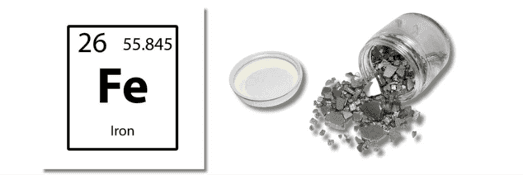
Iron, symbolized as Fe, is a heavy, lustrous, silver-gray metal that is the fourth most abundant element in the Earth’s crust. It is widely used in various industries and is essential for biological processes.
Physical Properties:
Iron has a high density of 7.9 g/cc, making it a heavy metal. It has a shiny, grayish-white color and is highly malleable and ductile. It is also a good conductor of heat and electricity and can be magnetized. Iron has a melting point of 1536°C and a boiling point of 2861°C. It readily dissolves in dilute acids.
Chemical Properties:
Iron is a chemical element that has several key properties. It has an atomic number of 26 and an electron configuration of [Ar] 3d6 4s2. It exists in four different crystalline forms depending on temperature.
One of the most well-known properties of iron is its reactivity. It readily reacts with oxygen to form iron oxide, commonly known as rust. This reactivity is also seen when iron combines with other nonmetals, such as chlorine.
Iron can exist in different oxidation states, with +2 and +3 being the most common. This is important in redox reactions.
Iron is an essential element for all forms of life. It plays a crucial role in hemoglobin, which carries oxygen in our blood, and in various enzymes. Iron deficiency can lead to anemia, while excess iron can cause health problems. In the environment, iron is persistent and can be harmful to plants, air, and water.
Iron possesses several unique characteristics that make it an exceptional element. These include its nuclear stability, abundance, biological significance, versatility in alloys, and reactivity. These properties collectively contribute to iron’s crucial role in various industries, technological advancements, and the natural environment.
Reactivity: Iron is highly reactive, combining vigorously with chlorine and other nonmetals. However, it is also susceptible to corrosion and rusting when exposed to air and water.

Biological Functions: Iron plays a vital role in the human body, as it is a key component of hemoglobin, which transports oxygen in the blood. It is also important for plant growth and development.

When comparing iron to steel, the key differences are that steel is an alloy of iron and carbon, with improved strength, malleability, and a lower melting point compared to pure iron. These differences make steel a more versatile and widely used material compared to pure iron.

1. Composition:
Iron is a pure metallic element, while steel is an alloy composed primarily of iron, carbon, and other alloying elements.
2. Carbon Content:
Steel contains less than 2% carbon, while cast iron contains 2-4% carbon.
3. Strength and Durability:
Steel is generally stronger and more durable than pure iron, due to the addition of carbon and other alloying elements.
Steel has higher compressive strength, while cast iron has higher tensile strength.
4. Malleability and Ductility:
Steel is more malleable and ductile than pure iron, allowing it to be easily shaped and formed.
Pure iron is more brittle compared to steel.
5. Melting Point:
The melting point of steel (around 2,500°F) is lower than the melting point of pure iron (around 2,800°F).
6. Applications:
Steel has a wider range of applications, including construction, transportation, machinery, and household items.
Pure iron has more limited applications, often requiring alloying to improve its properties.
Iron tools are a common part of our everyday lives. To ensure that they remain in good condition, it is important to take proper care of them. Here are some tips to help you keep your iron tools in top shape.
Regular Cleaning:
Rust & Corrosion Prevention:
Temperature & Lubrication:
Inspection & Maintenance:
Proper Storage & Handling:
Following these practices can extend the lifespan and performance of your iron tools.

Iron has been a vital component of human civilization for thousands of years. It is used in a wide range of applications, from constructing strong buildings to carrying oxygen in our blood. Its versatility and essential role demonstrate why iron will continue to play a crucial part in advancing technology and biology. The story of iron is not yet complete; it remains as significant today as it was in ancient times.
For pure iron and customized iron products, MetalsTek can always be your trusted source.