Iron II sulfide, also known as ferrous sulfide, is a chemical compound that plays a significant role in various industrial applications. Understanding its chemical formula is crucial for comprehending its composition and unlocking its potential uses. In this essential guide, we will delve into the world of iron II sulfide, decoding its formula and shedding light on its diverse applications.
The chemical formula of iron II sulfide can be written as FeS, which indicates the presence of one iron atom (Fe) and one sulfur atom (S). This simple composition gives iron II sulfide its distinct properties, making it a valuable compound in fields such as metallurgy, chemical manufacturing, and the production of iron alloys.
Discover how the unique characteristics of iron II sulfide enable it to be used as a lubricant, for pigment production, and in the creation of materials with magnetic properties. We will also explore its role in the production of sulfuric acid and its significance in the oil and gas industry.
Join us as we unlock the secrets of iron II sulfide and uncover its wide-ranging applications in industries around the world.

Chemical Composition of Iron II Sulfide
Iron II sulfide, represented by the chemical formula FeS, consists of one iron atom bonded to one sulfur atom. This composition results in a compound that exhibits distinct characteristics, including its blackish color and metallic luster. The atomic arrangement within iron II sulfide contributes to its stability and reactivity, making it a valuable component in various chemical processes. Understanding the precise composition of FeS is crucial for harnessing its potential benefits in industrial applications.
Iron II sulfide is commonly synthesized through the reaction between iron and sulfur elements under controlled conditions. This process allows for the formation of FeS with the desired purity and properties. The chemical bonding between iron and sulfur atoms in the compound determines its physical and chemical behavior, influencing its suitability for specific uses. By examining the composition of iron II sulfide at the atomic level, researchers can unlock new insights into its structure-function relationships and potential applications.
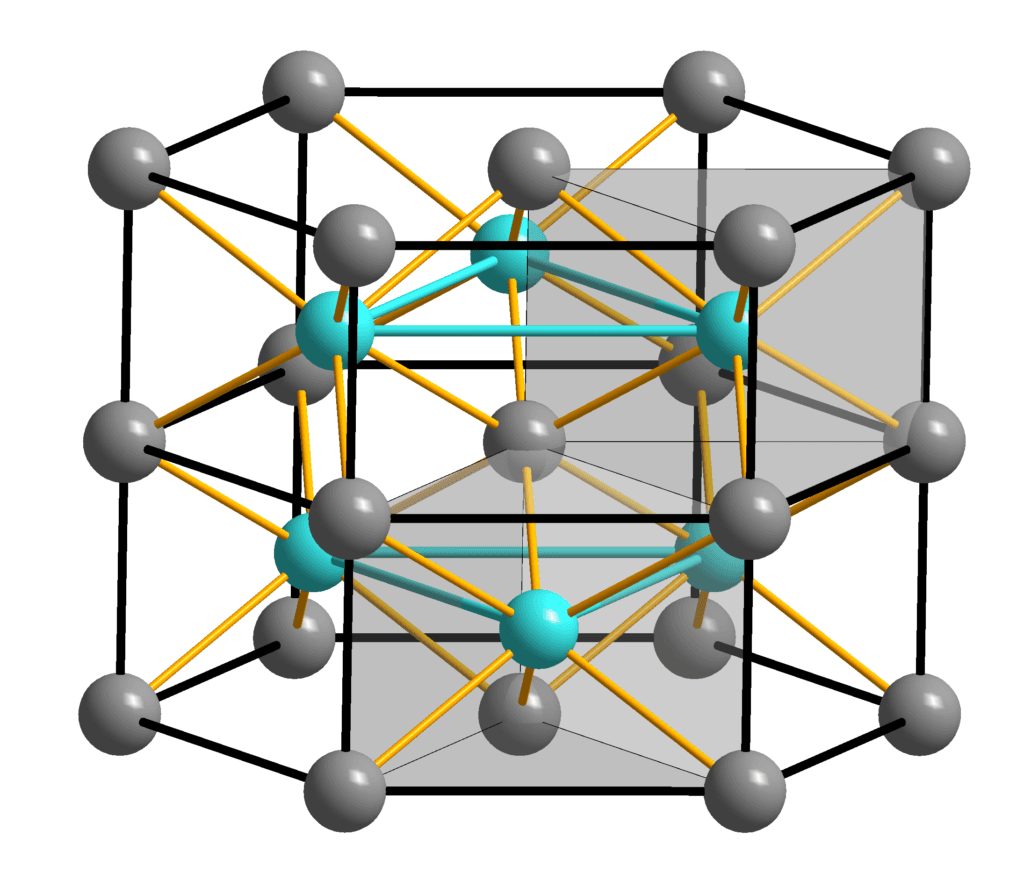
**Quoted from Wikipedia
Properties of Iron II Sulfide
Iron II sulfide possesses several key properties that make it a valuable material in various industrial applications. One of the notable characteristics of FeS is its semiconducting behavior, which enables it to conduct electricity under specific conditions. This property is exploited in the production of electronic devices and components that require semiconducting materials. Additionally, the magnetic properties of iron II sulfide make it useful in the manufacturing of magnets and magnetic materials.
The black color of iron II sulfide is attributed to its absorption and reflection of light in the visible spectrum. This property has implications for its use as a pigment in the production of paints, dyes, and coatings. Furthermore, the lubricating properties of FeS make it suitable for applications where reducing friction and wear between moving parts is essential. By understanding the unique properties of iron II sulfide, researchers and engineers can optimize its use in various industrial processes.
Applications of Iron II Sulfide
Iron II sulfide finds applications in diverse industries, thanks to its unique properties and chemical composition. In the field of metallurgy, FeS is used as a precursor for the production of iron and steel alloys. Its ability to improve the mechanical properties of metals makes it a sought-after additive in the manufacturing of structural materials. Additionally, iron II sulfide is employed in the production of sulfuric acid, a key chemical used in various industrial processes.
The lubricating properties of iron II sulfide make it a valuable component in the formulation of lubricants and greases for machinery and equipment. Its ability to reduce friction and wear on moving parts enhances the efficiency and longevity of mechanical systems. Furthermore, FeS is utilized in the oil and gas industry for various applications, including drilling fluids and corrosion inhibition. By exploring the diverse applications of iron II sulfide, we can appreciate its importance across multiple sectors.
Production Methods of Iron II Sulfide
The production of iron II sulfide involves several methods that vary in complexity and scalability. One common approach is the direct reaction between iron and sulfur elements at elevated temperatures, resulting in the formation of FeS. This method is suitable for laboratory-scale synthesis and small-scale production of iron II sulfide. Another technique involves the chemical precipitation of iron and sulfur compounds to yield FeS with controlled particle size and purity.
In industrial settings, iron II sulfide can be obtained through pyrometallurgical processes that involve the smelting of iron ores containing sulfur impurities. The extraction of FeS from these ores requires specialized equipment and facilities to ensure the efficient separation of iron and sulfur components. By optimizing production methods for iron II sulfide, manufacturers can meet the demand for this versatile compound in various applications.
Safety Considerations When Handling Iron II Sulfide
While iron II sulfide offers numerous benefits in industrial applications, it is essential to consider safety precautions when handling this compound. Due to its reactivity with certain chemicals and substances, FeS may pose hazards if not handled properly. Workers involved in the production, storage, and transportation of iron II sulfide should be equipped with appropriate personal protective equipment to minimize exposure.
In addition, proper ventilation and containment measures should be implemented in areas where FeS is processed or stored to prevent the buildup of hazardous concentrations. Regular safety training and emergency response procedures should be in place to address any potential accidents or incidents involving iron II sulfide. By prioritizing safety considerations in the handling of FeS, industries can ensure the well-being of workers and the surrounding environment.
Environmental Impact of Iron II Sulfide
The environmental impact of iron II sulfide primarily stems from its potential to release sulfur compounds into the air and water during manufacturing and usage. Sulfur emissions can contribute to air pollution and acid rain, affecting ecosystems and human health. Proper management of FeS waste products and byproducts is crucial to mitigate environmental contamination and minimize adverse effects on the environment.
Efforts to reduce the environmental footprint of iron II sulfide production include implementing pollution control technologies, recycling waste materials, and adopting sustainable practices. By incorporating eco-friendly approaches in the synthesis and utilization of FeS, industries can reduce their environmental impact and contribute to sustainable development. Understanding the environmental implications of iron II sulfide is essential for promoting responsible use and disposal practices.
Comparing Iron II Sulfide with Other Sulfides
Iron II sulfide belongs to a family of sulfide compounds that exhibit diverse properties and applications. A comparative analysis of FeS with other sulfides, such as zinc sulfide and copper sulfide, reveals differences in their chemical compositions, physical properties, and uses. While zinc sulfide is known for its luminescent properties and applications in optoelectronic devices, copper sulfide is valued for its conductivity and catalytic properties.
Each sulfide compound offers unique advantages and challenges in various industrial applications, depending on the specific requirements of the process or product. By comparing the properties and performance of different sulfides, researchers and engineers can select the most suitable compound for a given application. Understanding the distinctions between iron II sulfide and other sulfides provides valuable insights into their respective roles in industry and technology.
Future Prospects and Developments in Iron II Sulfide Research
The field of iron II sulfide research continues to evolve, with ongoing efforts to explore new applications, improve production methods, and enhance the properties of FeS-based materials. Advances in nanotechnology have opened up exciting possibilities for utilizing iron II sulfide nanoparticles in various technological innovations, such as sensors, catalysts, and energy storage devices. The tunable properties of FeS nanoparticles offer versatile solutions for diverse industrial challenges.
Furthermore, research into the catalytic properties of iron II sulfide has shown promise in environmental remediation and sustainable energy production. By leveraging the unique reactivity of FeS in catalytic reactions, researchers aim to develop efficient processes for reducing pollutants and generating clean energy sources. The interdisciplinary nature of iron II sulfide research paves the way for novel applications and discoveries that could shape the future of materials science and technology.
Conclusion
In conclusion, the chemical formula of iron II sulfide, FeS, encapsulates the essence of a compound with diverse properties and applications. By understanding the composition, properties, and uses of FeS, we can appreciate its significance in metallurgy, chemical manufacturing, and various industrial sectors. From lubricants to pigments, from magnets to catalysts, iron II sulfide plays a crucial role in shaping modern technologies and processes.
As research and development efforts continue to unravel the mysteries of iron II sulfide, new opportunities emerge for harnessing its potential in emerging fields and applications. By staying informed about the latest advancements in FeS research and technology, we can pave the way for innovative solutions and sustainable practices that leverage the unique properties of this versatile compound. The journey of decoding the chemical formula of iron II sulfide is an ongoing exploration of its vast potential and endless possibilities in the world of materials science and technology.

One Reply to “The Essential Guide to the Chemical Formula of Iron II Sulfide: All You Need To Know”
I like this.