Electron-beam (E-beam) evaporation is one of the workhorses of modern physical vapor deposition (PVD). From semiconductor wafers and optical components to advanced sensors and aerospace hardware, it is relied on whenever high-purity, dense, and well-controlled thin films are required. Inside every E-beam source sits a deceptively simple but absolutely critical component: the crucible or liner that holds the evaporation material under intense thermal and electron bombardment.
Among the various crucible materials available—tungsten, molybdenum, tantalum, alumina, boron nitride, pyrolytic boron nitride—graphite E-beam crucibles have emerged as a very practical choice. They offer a unique balance of thermal performance, stability, cost, and compatibility with a wide range of metals, alloys, and compounds. For engineers running production coaters and buyers responsible for keeping those coaters supplied, understanding what graphite crucibles can and cannot do is essential.
This article provides a deeper look at graphite E-beam crucibles: why they work, how they are manufactured, how they behave under real operating conditions, and where they fit compared with alternative crucible materials. It is written as a technical buyer’s guide for users of systems from Telemark, Temescal, Lesker, Denton, CHA, ULVAC and custom OEM chambers, as well as R&D labs that frequently change materials and processes.
Graphite Crucibles in Modern E-Beam Evaporation
In an E-beam source, a focused beam of high-energy electrons strikes a solid charge of metal, oxide, fluoride, nitride or more complex material. The beam’s kinetic energy is converted to heat, and the surface of the charge melts and evaporates into the vacuum chamber. The substrate, positioned above the source, collects this vapor as a thin film.
Because the E-beam is so localized, the region under the beam can reach temperatures of 2000–3000 °C or higher. The crucible must withstand:
- Very high temperatures
- Steep thermal gradients between the hot melt and the cooler hearth
- Rapid power changes and thermal cycling
- Chemical interaction with molten materials and background gases
The crucible is therefore not a passive container. It directly affects deposition rate stability, film purity, material utilization efficiency, and even the lifetime of the E-beam gun and copper hearth. If the crucible cracks, reacts strongly with the charge, or sheds contaminants, the entire coating run is compromised.
Graphite crucibles address many of these requirements simultaneously. They are machined from high-density, high-purity graphite blocks and shaped into pockets, wells, steps, or inserts that drop into the water-cooled copper hearth. Metalstek supplies these crucibles in standard geometries compatible with common multi-pocket sources, as well as custom liners matched to OEM or special-purpose systems. In production environments, operators appreciate graphite because it is robust enough to survive repeated cycles, yet economical enough that liners can be replaced as a routine consumable rather than a capital part.
Material Properties That Make Graphite Crucibles Work
The performance of graphite E-beam crucibles is rooted in the structure of graphite itself. Graphite is composed of carbon atoms arranged in hexagonal layers. These layers slide over each other easily (hence its use as a lubricant), but they also form a stable, high-temperature solid with interesting thermal and chemical behavior.
One of the most important properties for PVD is graphite’s high thermal conductivity. Typical dense isostatic graphite used for crucibles has a thermal conductivity in the range of roughly 80–150 W/m·K, several times higher than alumina. In practical terms, this means heat from the E-beam does not stay in a small hot spot; it spreads through the crucible wall, smoothing out temperature gradients. A more uniform temperature in the melt pool translates into more stable evaporation, fewer spitting events, and less foaming or violent boiling of the charge.
Graphite is also exceptionally refractory. It does not have a conventional melting point; instead it sublimates above approximately 3650 °C. In a vacuum chamber, a well-cooled graphite crucible can tolerate the very high local temperatures under the E-beam without softening or collapsing. This temperature margin is one reason graphite can be used with a wide range of materials, from low-melting-point metals such as indium and tin all the way up to refractory metals and carbides.
Thermal shock resistance is another major advantage. Many ceramics, including alumina, crack when subjected to rapid heating and cooling. Graphite, by contrast, can handle fast power ramps and frequent cycling. Its combination of high thermal conductivity, relatively low thermal expansion, and inherent toughness in compression allows it to survive the harsh on/off duty cycles typical of production and R&D.
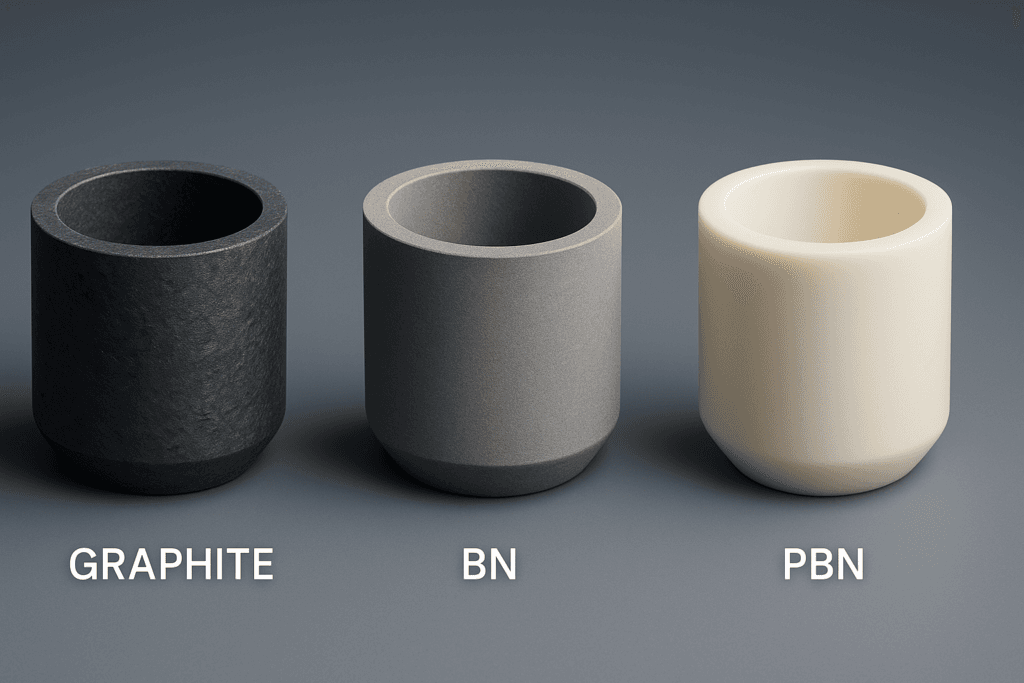
Chemically, graphite is inert to many metals in a high-vacuum, oxygen-lean environment. Noble metals, most transition metals, rare-earth metals, and many alloys wet graphite moderately and evaporate cleanly without strong reaction. Graphite also tolerates fluorides and a number of nitrides and sulfides. Problems arise primarily in two situations: strongly oxidizing atmospheres at high temperature, where graphite can oxidize to CO/CO₂, and with certain oxides that are easily reduced, which can pick up carbon or convert to carbides. These cases can be managed with coated crucibles or by selecting alternative materials such as BN or PBN for specific processes.
From a contamination standpoint, the key is purity. High-quality crucibles are made from high-purity isostatic graphite with controlled ash content (often below 20 ppm). This minimizes the risk of metallic impurities entering the film. For more demanding work—high-end optics, advanced semiconductor processes—Metalstek can supply premium grades with even higher purity and tighter control over trace elements.
Mechanically, graphite is not as strong as metals and it is brittle under bending or impact, but in compression and at elevated temperature it performs well. Once installed in the hearth and properly supported, a graphite crucible easily maintains its shape through many runs. Operators mainly need to avoid rough mechanical handling—dropping crucibles or prying at them with hard tools—because that can chip edges or initiate cracks.
From Raw Graphite Block to Finished Crucible
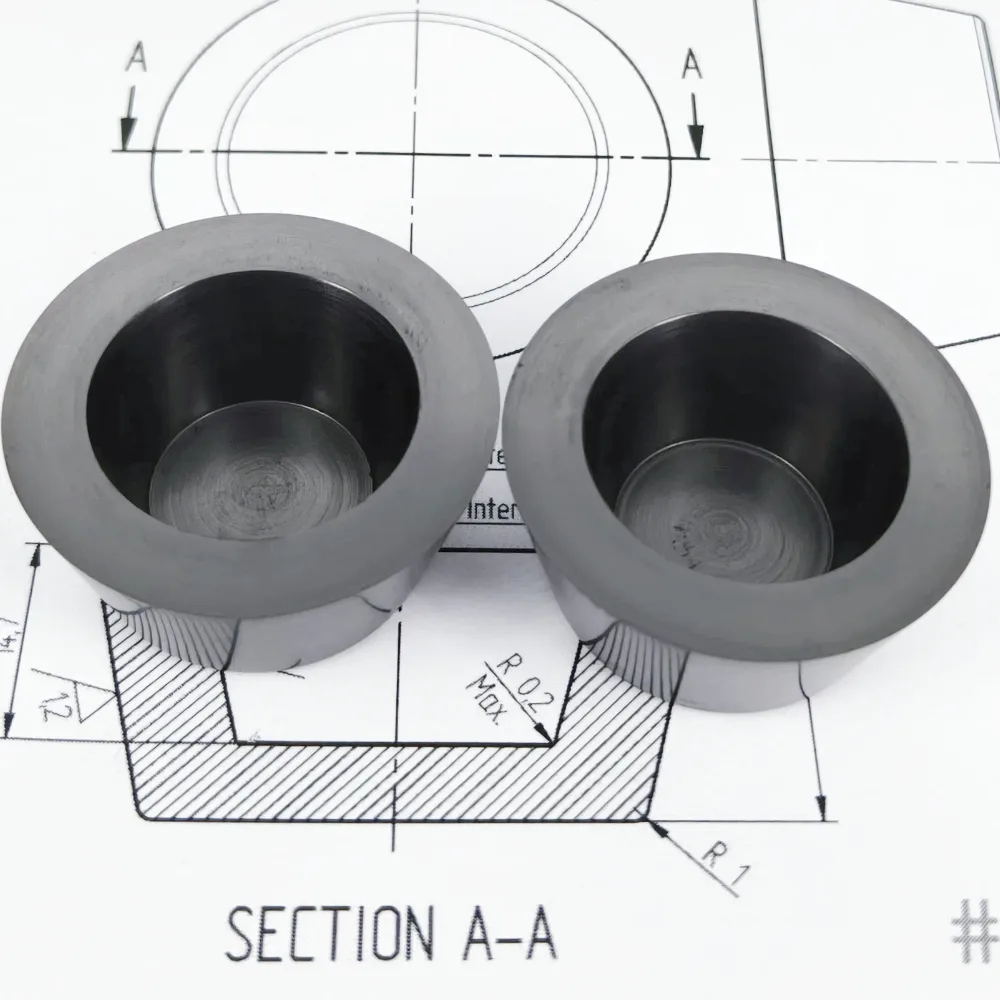








Manufacturing an E-beam crucible begins with choosing the right graphite. For demanding thin-film applications, Metalstek uses high-density isostatic graphite with fine grain size and uniform porosity. This ensures consistent thermal behavior, predictable machining, and minimal internal defects. The raw blocks are inspected for density and composition before they ever reach a CNC machine.
Machining is carried out on dedicated equipment, using tools and parameters optimized for graphite. Because graphite is relatively soft and dusty, cutting strategies differ from metal machining; dust extraction and cleanliness are important to avoid contamination. The machinability of graphite is a major advantage: it is straightforward to produce precise cylindrical pockets, flat-bottom wells, conical shapes, tapered bores, and complex hearth liners with features such as steps, lips, or shoulders that lock into a copper hearth.
This is what allows Metalstek to supply crucibles that drop directly into common multi-pocket designs from Telemark, Temescal, Lesker and others, as well as custom liners that follow proprietary OEM geometries. Tolerances are kept tight so that the crucible sits firmly in the hearth, ensuring good thermal contact and stable positioning under the beam. Surface finish is also controlled; a smooth interior helps the melt pool behave more predictably and can simplify cleaning between runs.
Optionally, an additional coating step can be applied. For example, a thin boron nitride (BN) coating can be deposited on the interior surface to increase chemical inertness and reduce wetting by aggressive oxides. Pyrolytic graphite or silicon carbide coatings are also used in some processes to add a diffusion barrier or further reduce interaction between the charge and the crucible body. These coatings are thin enough not to compromise thermal performance but can extend crucible life and reduce carbon pickup for sensitive materials.
Quality control does not stop at dimensions. Finished crucibles are inspected for visible defects, checked against drawings, and, for high-purity applications, sampled for ash content and trace impurities when required. Porosity and density are verified so that heat conduction and outgassing behavior remain consistent from lot to lot. For customers running tightly controlled coating recipes, this consistency is just as important as basic dimensional accuracy.
Practical Advantages, Limits, and Compatibility in Real Use
For most customers, the first thing they notice about graphite E-beam crucibles is cost. Compared with refractory metals such as tungsten and molybdenum, graphite is significantly less expensive. A graphite liner that lasts tens or even hundreds of runs before replacement can dramatically reduce consumable cost per deposition, especially in high-throughput coaters. When multiple materials are used, separate graphite liners can be kept for each family of materials rather than cleaning a single expensive metal hearth over and over.
The second advantage is process stability. Because graphite conducts heat efficiently, the molten pool tends to be more uniform and less prone to localized overheating. With proper beam sweeping and power control, this leads to smoother evaporation fronts, more consistent deposition rates, and fewer violent events such as spitting or splashing. Many process engineers find that moving from a low-conductivity ceramic liner to graphite improves thickness uniformity across substrates and reduces the number of aborted runs.
Compatibility with many common evaporants makes graphite a natural default choice. Aluminum, copper, silver, gold, nickel, zinc, tin, indium, lead, platinum-group metals, titanium, zirconium, hafnium and most rare-earth metals can be evaporated from graphite crucibles with good results. Fluoride materials such as MgF₂, CaF₂ and LiF also run well, and carbides or some nitrides can be processed successfully depending on the exact chemistry and temperature profile. For routine metal coatings, the graphite crucible is often treated as a standard consumable with predictable lifetime.
There are, however, important limits. Some metal oxides and certain reactive materials can reduce in contact with graphite at high temperature, leading to unwanted carbon incorporation or phase changes. Chromium oxides, titanium oxides, and specific high-temperature oxides are typical examples. In these cases, the process engineer must either accept some degree of reaction, move to coated graphite (for example BN-coated liners), or choose alternative crucible materials like BN, PBN or refractory metals. Similarly, graphite should not be exposed to high temperature in the presence of oxygen, though in a high-vacuum E-beam chamber under normal operation this is not a concern.
Mechanical fragility is another consideration. Graphite is brittle compared with metals, so crucibles should be handled carefully during installation and removal. Most damage is caused not by the E-beam, but by accidental drops or by using metal tools to pry a stuck crucible out of the hearth. Careful handling and good housekeeping in the evaporation area usually prevent these problems.
Lifetime depends strongly on the evaporant and operating conditions. For low-melting metals run at modest power, a graphite crucible may last well over one hundred cycles before signs of wear appear. For high-temperature refractory metals or processes that demand very aggressive power ramps, wear and erosion come sooner. Where aggressive fluorides or corrosive materials are used, coatings can extend lifetime significantly. In practice, many users monitor the condition of crucibles visually and through their process data: when the behavior of the melt or deposition rate begins to deviate, they schedule crucible replacement as preventive maintenance.
Applications, Material Choices, and Selection Criteria
Graphite E-beam crucibles are used across almost every sector that employs PVD. In semiconductor manufacturing, they are common in metallization lines for depositing aluminum and copper interconnects, diffusion barriers, seed layers, and contact structures. Because semiconductor fabs often use complex multi-pocket hearths, graphite liners allow fast material changeover and help protect the expensive copper hearth from direct attack by the melt.
In optical coating facilities, graphite crucibles handle both pure metals and many of the compounds used in multilayer stacks. Reflective aluminum mirrors, silver and gold layers for infrared and laser optics, and certain fluoride layers for anti-reflection or bandpass filters can all be evaporated from graphite. For oxides that are sensitive to reduction or carbon pickup, operators may switch to BN or PBN crucibles for those specific steps while continuing to use graphite for the remaining metals and stable compounds. This mix-and-match approach keeps material cost under control.
Energy and solar applications also rely on graphite crucibles for metals such as indium, copper, silver and aluminum in thin-film solar cells and other device structures. In research laboratories, where materials change frequently and process recipes are constantly being adjusted, graphite’s versatility and cost-effectiveness are particularly attractive. It allows researchers to experiment with new compositions without committing to expensive noble-metal or high-end ceramic liners for every trial.
When selecting a crucible, engineers typically start from the evaporant. If the material is a relatively benign metal or fluoride that does not react strongly with carbon, graphite is usually an excellent choice. They then consider system geometry—Telemark, Temescal, Lesker, Denton, custom OEM—and pick a crucible shape that matches the hearth pockets and beam steering pattern. Pocket depth, diameter, and taper are chosen to achieve the desired charge volume and melt surface profile. For multi-material processes, multi-pocket graphite crucibles may be specified, enabling rapid switching under vacuum.
Power and temperature requirements come next. Very high melting-point materials or extremely high fluxes may push the crucible to its limits. In some of these extreme cases, refractory metal crucibles or hybrids may be recommended. Purity requirements are also taken into account: for general industrial coatings, standard 99.9 % graphite is often sufficient; for more sensitive optical or microelectronic applications, a 99.99 % or higher-purity grade may be selected.
Budget, of course, is an ever-present constraint. Compared with tungsten, molybdenum, BN and especially PBN, graphite is almost always the most economical option. For cost-sensitive production lines, the ability to standardize on graphite for the majority of processes and reserve higher-end materials only for the most critical steps can significantly reduce operating expenses.
Metalstek supports this selection process by offering standard graphite crucibles compatible with common E-beam sources, as well as fully custom CNC-machined crucibles and liners. Coated options—BN-coated graphite, pyrolytic graphite-coated, silicon carbide-coated—are available for processes where additional chemical inertness or longer lifetime is needed. Typical lead times for standard parts are short, and custom designs can often be implemented within a few weeks, depending on complexity.
Conclusion and Contact
Graphite E-beam crucibles occupy a very useful middle ground in thin-film production: they are robust enough to withstand extreme thermal conditions, thermally conductive enough to stabilize melts and evaporation rates, chemically compatible with a wide range of metals and compounds, and economical enough to treat as consumables in high-throughput coating lines. When properly selected and handled, they contribute directly to better film uniformity, lower contamination, more predictable process control, and lower cost of ownership.
For engineers and buyers planning new E-beam lines or optimizing existing ones, graphite crucibles should almost always be part of the conversation. They are ideal as general-purpose liners for metals and many compounds, and when combined with selective use of BN, PBN, or refractory metal crucibles for especially reactive materials, they form the backbone of a flexible and cost-efficient crucible strategy.
Metalstek supplies high-purity, precision-machined graphite E-beam crucibles in both standard formats and custom geometries tailored to your evaporator design and process requirements. If you are evaluating a new coating recipe, facing film stability issues, or simply want to reduce consumable costs while maintaining film quality, our team can help you choose the most appropriate crucible materials and designs for your application.
For detailed specifications, engineering support, or a quotation on graphite E-beam crucibles and related evaporation components, please contact:
Email: sales@metalstek.com
Website: https://www.metalstek.com
